Abstract
Background: The Total Thrombus Formation Analysis System (T-TAS) is a new approach proposed to explore hemostasis in samples with low platelet counts. T-TAS may become an alternative tool to explore the hemostatic effect of platelet products and platelet substitutes.
Aims: To validate T-TAS as a suitable device to evaluate hemostasis in samples from thrombocytopenic patients before and after platelet transfusion. To explore the hemostatic efficiency of in vitro addition of Thrombosomes®, a human platelet-derived lyophilized hemostatic preparation proposed for the treatment of bleeding in thrombocytopenia. To investigate the mechanisms involved in the hemostatic action of these lyophilized preparations using confocal microscopy in microfluidic chambers.
Methods: Whole blood from hematological thrombocytopenic patients (platelet count <30x103 platelets/µl) was collected before (N=31) and after platelet transfusion (N=19). Hemostasis was evaluated through T-TAS, with specific chips (HD) containing microcapillary channels (50-μm-deep) coated with collagen and tissue factor. Area under the curve (AUC) and occlusion times (OT, min) were registered for all samples before and after addition of Thrombosomes® (50x103/µl). For confocal studies, thrombocytopenic blood was circulated through microfluidic chambers coated with collagen and tissue factor. A triple labelling procedure was used to identify and quantify interactions of platelets, Thrombosomes® or fibrin in microfluidic studies.
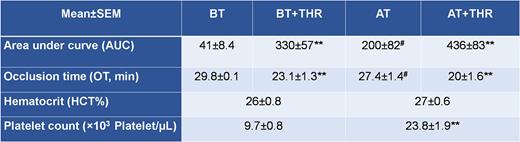
Results: Values (Mean±SEM) of platelet counts were of 9.7±0.8 and 23.8±1.9 (x103 platelets/µl) and hematocrits (HCT) were of 26±0.8% and 27±0.6%, before and after platelet transfusion, respectively. T-TAS parameters improved significantly after platelet transfusion (Table 1). Addition of Thrombosomes® increased AUC and shortened OT in 60% and 100% of samples from non-transfused (p˂0.01) and transfused patients, respectively. In confocal studies, Thrombosomes® associated with platelets interacting with the thrombogenic substrata and potentiated fibrin formation.
Conclusions: In flow studies under thrombocytopenic conditions, Thrombosomes® participated with the available platelets to form aggregates and support coagulation in a manner like that of native blood. The T-TAS system was able to measure clot formation in a range of simulated flow conditions in samples of blood from thrombocytopenic patients. Thrombosomes® enabled adherent clot formation even when samples had initial T-TAS parameters severely impaired. The system may prove useful to evaluate key hemostatic properties of transfused platelets or Thrombosomes®. Additional clinical verification will be needed to confirm the clinical utility of the T-TAS assay system.
Table 1. T-TAS parameters (AUC and OT; Mean ± SEM) obtained in samples from thrombocytopenic patients before (n=31) and after (n=19) platelet transfusion. In vitro effect of Thrombosomes® (THR).
BT= before transfusion, AT= after transfusion
**p<0.01 for the effect of the presence of Thrombosomes ® vs. their absence, in each sample
#p<0.05 after transfusion vs. before transfusion (AUC = 28±2) in the n=19 patients
Disclosures
Moskowitz:Cellphire Therapeutics Inc: Current Employment, Current holder of stock options in a privately-held company. Alexander:Cellphire Therapeutics Inc: Current Employment, Current holder of stock options in a privately-held company. Diaz-Ricart:Cellphire Therapeutics Inc: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal